Di-Fluoro ethylene carbonate:Application And Chemical Studies
General Description
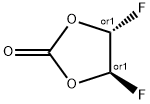
Di-Fluoro ethylene carbonate, its molecular weight is 124.04 and chemical name is (4R,5R)-4,5-difluoro-1,3-dioxolan-2-one. Due to the advantages of high dielectric constant, high flash point, low freezing point and good oxidation resistance, di-fluoro ethylene carbonate can significantly improve the cycle performance, high temperature performance and flame retardant performance of the electrolyte, and is an ideal lithium battery electrolyte additive. At present, the amount of di-Fluoro ethylene carbonate is small, but its excellent performance is gradually being favored by major manufacturers, and the market prospect is good.[1]

Figure 1 Di-Fluoro ethylene carbonate solution
Preparation
Chlorinated ethylene carbonate is a common raw material for the preparation of vinylene carbonate and di-fluoro ethylene carbonate. In industry, chlorine gas and ethylene carbonate are used for substitution reaction to prepare chloroethylene carbonate, and the by-product is di-fluoro ethylene carbonate.The preparation of di-fluoro ethylene carbonate into electronic grade di-fluoro ethylene carbonate can greatly improve the To improve the yield of products, reduce waste and realize green process. And it can reduces the amount of hazardous waste and environmental protection cost, and has simple process and low energy consumption, and is suitable for large-scale production,application.The preparation method is as follows.[1]
First method:Under nitrogen protection, add 348g of highly active anhydrous potassium fluoride and 560ml of dimethyl carbonate after molecular sieve dehydration into a 3000ml four-necked flask, connect the condenser vent pipe and other devices, turn on stirring, turn on heating, and heat up to 70° C., add 400g of ethylene dichlorocarbonate (purity 98%) dropwise with a constant pressure dropping funnel, dropwise for 2 hours, after the dropwise addition, keep the reaction for 1 hour, cool down and filter and wash the flask with 140ml of dimethyl carbonate and filter residue to obtain 1kg of filtrate. The obtained filtrate was transferred to a distillation apparatus, the solvent was recovered by atmospheric distillation, and the solvent was recovered to obtain 510 g of dimethyl carbonate. The remaining filtrate continued to be distilled under reduced pressure, and the distillation was completed to obtain a product of 320.9 g of di-fluoro ethylene carbonate, with a GC content of 87.62%. Further rectification and purification were carried out to obtain 290 g of high-purity ethylene difluorocarbonate with a GC content of 97.65%. Crystallization sweating is used to purify to obtain higher-purity di-fluoro ethylene carbonate, and 278 g of high-purity di-fluoro ethylene carbonate can be obtained after repeated crystallization and sweating, with a GC content of 99.99% and a yield of 89.78%.[1]
Second method:Under nitrogen protection, add 348g of highly active anhydrous potassium fluoride and 560ml of dimethyl carbonate after molecular sieve dehydration into a 3000ml four-necked flask, connect the condenser vent pipe and other devices, turn on stirring, turn on heating, and heat up to 80° C., add 400g of ethylene dichlorocarbonate (purity 98%) dropwise with a constant pressure dropping funnel, dropwise for 2 hours, after the dropwise addition is completed, the reaction is incubated for 1 hour, cooled and filtered and the flask is washed with 140ml of dimethyl carbonate and filter residue to obtain 1kg of filtrate. The obtained filtrate was transferred to a distillation apparatus, the solvent was recovered by atmospheric distillation, and the solvent was recovered to obtain 490 g of dimethyl carbonate. The remaining filtrate continued to be distilled under reduced pressure, and the distillation was completed to obtain a product of 302 g of di-fluoro ethylene carbonate, with a GC content of 85.43%. Further rectification and purification to obtain 273 g of high-purity ethylene difluorocarbonate with a GC content of 97.52%. Crystallization sweating was used to purify to obtain higher-purity di-fluoro ethylene carbonate, and 245 g of high-purity di-fluoro ethylene carbonate could be obtained after repeated crystallization and sweating, with a GC content of 99.99% and a yield of 79.13%.[1]
Application
Di-fluoro ethylene carbonate, synthesized by further fluorination of FEC, is another emerging film-forming agen.What's more, it can be used in the manufacture of batteries.[2]
Reference
[1]Wang Y,Jiang F,Zhai T,et al.Preparation of electronic grade di-fluoro ethylene carbonate[P].2022,CN114014834.
[2]Piao Z, Gao R, Liu Y, et al. A review on regulating Li+ solvation structures in carbonate electrolytes for lithium metal batteries[J]. Advanced Materials, 2022: 2206009.